Whitley Anaerobic Isolators for Live Biotherapeutic Products

Supporting Global Innovators
 |  |  |
What our customers have to say ...
| |
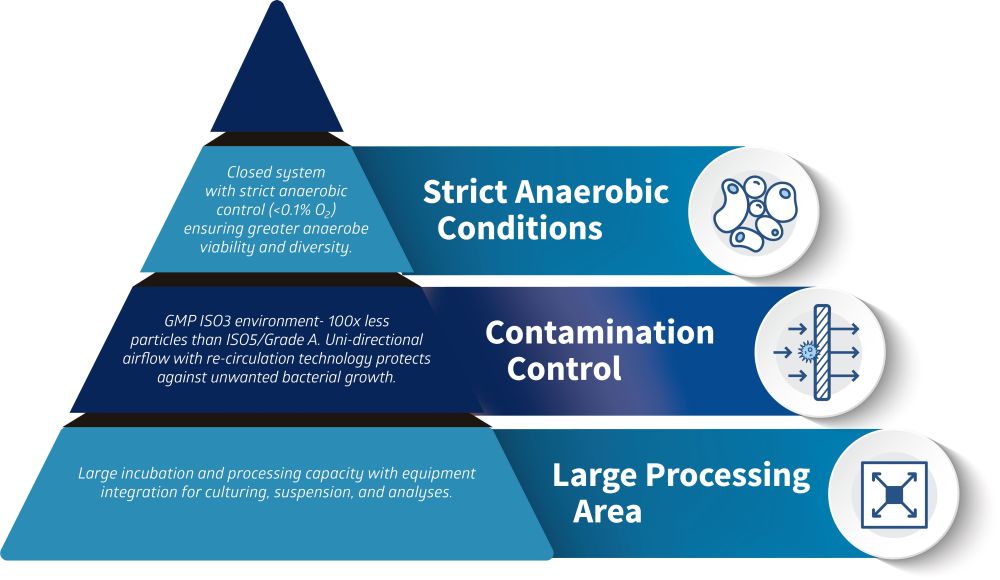
The Whitley Advantage
Modular Solutions
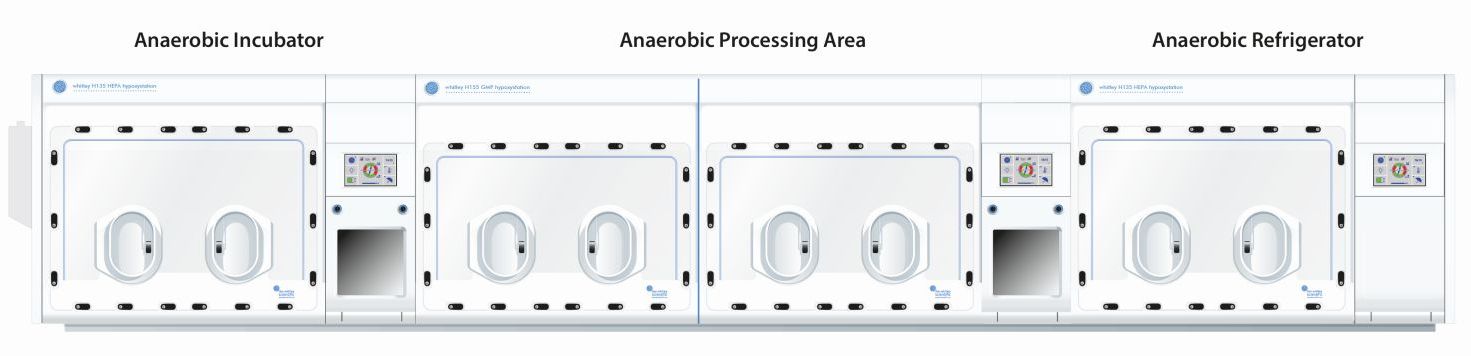
Equipment integrations:
- Microplate spectrophotometer
- Live cell microscopes
- Mini bioreactors
- Centrifuges
- Lyophilizers
Who we are
Don Whitley Scientific has been manufacturing modified atmosphere workstations for almost half a century.
Empowering leading microbiome manufacturers around the world.
After years of partnership with leading live biotherapeutic companies, our third-generation, family-run approach is logical: deliver the highest quality anaerobic technologies with all the support you need, because we can.



 en
en


 xEnglish
xEnglish